The cynical side of me always wonders about the actual content of those vaccine vials that are used when politicians and other high profile people get their COVID-19 vaccines as part of a publicity stunt. Perhaps there is "nothing to see here, just move along" but recent information from Australia that was released as part of a Freedom of Information request suggests that all may not be what it appears to be. Thanks to a relatively recent Twitter thread by Jikkyleaks, we now have data from a Freedom of Information Request made to the Australian Government's Department of Health, Therapeutic Goods Administration back in December 2021 which has just been released which may help us to better understand the rollout of the COVID-19 vaccines.
On the Australian Government's Department of Health, Therapeutic Goods Administration's (TGA) website, we find the "Batch release assessment of COVID-19 vaccines" which the TGA uses to
"...ensure(s) there is an independent quality assessment of every batch vaccine supplied in Australia".
To ensure that manufacturing standards are maintained and to set up how the quality of the vaccine will be maintained in future batches, the batch assessment involves one or both of the following steps:
1.) A review of documents supplied by the Sponsor which describe the manufacturing process (how the vaccine is made, tested, shipped and stored).
2.) TGA laboratory testing (and/or review of testing results from an overseas regulatory laboratory that has been recognised by the TGA) to ensure the vaccine has been manufactured according to the required standards.
The TGA's laboratory may carry out a range of tests, including assessments for composition, identity, potency, purity and adventitious agents (contamination with microorganisms).
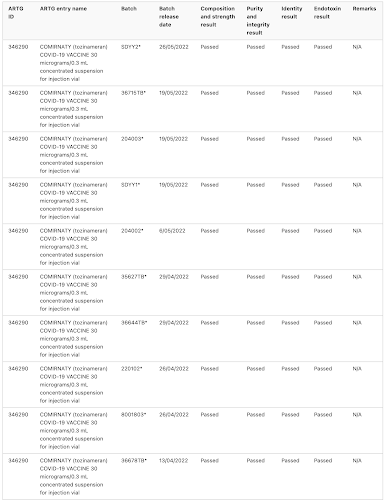
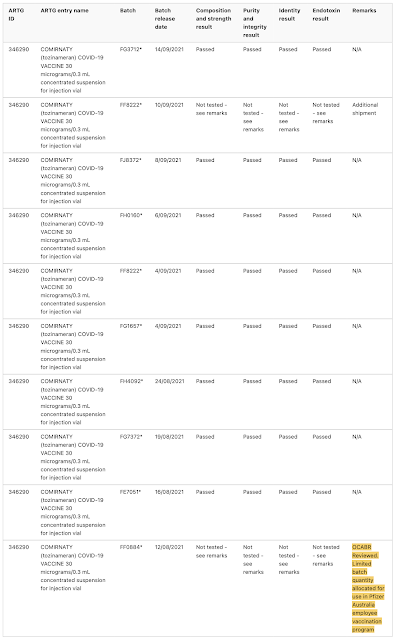
The TGA notes that batch release assessment is particularly important for provisionally approved vaccines like the COVID-19 vaccines and has provided the public with their assessment of 382 different batches of Pfizer's Comirnaty COVID-19 vaccine
This is what the TGA's batch assessment database looks like, noting that the database includes the manufacturer's batch number, what was tested and whether it passed:
Now, let's look at some interesting anomalies:
Batches FF0884, FA 4598FE3064, FA7338, FA7812, FC8736 and FC 3558 were (at least in part) reserved for use in Pfizer Australia's employee vaccination program, were all limited quantity batches that were not tested by TGA Laboratories, rather, the TGA accepted overseas certification from the Official Control Authority Batch Release or OCABR process in Europe. One would wonder why this would be the case. Can anyone explain why vials from these seven batches were reserved for Pfizer insiders?
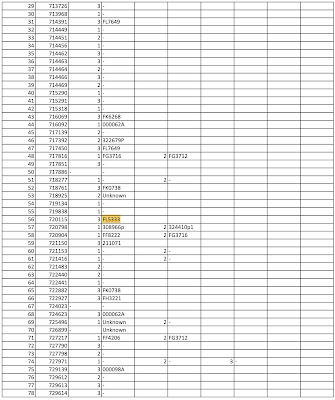
Perhaps we have part of the answer here which was released under Freedom of Information request 4077 dated December 6, 2021 as shown here:
...which reports dose batch number and deaths associated with each batch keeping in mind that this is only a small fraction of the total number of deaths reported to the TGA and that each row in the spreadsheet represents a person who died:
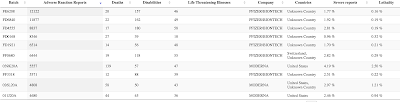
It looks like Comirnaty batch FL5333 was a particularly nasty one when it came to death after vaccination which was passed by TGA Laboratories on November 4, 2021 as shown here
I'll let you mull this information over and I'm open to any comments about whether or not we should all remain cynical about the veracity of the COVID-19 vaccination program for the global ruling class.






No comments:
Post a Comment