Over the past 20 months, it has become very apparent that social media outlets in specific and Big Technology in general have done their best to control the COVID-19 narrative, censoring any information that doesn't subscribe to their viewpoint, no matter how qualified the source of the information may be.
As a prime example of promoting the so-called "truth" , Twitter has recently amended its policies regarding COVID-19 "misleading information", noting in particular the highlighted section:
In fact, if you look back at an earlier version of the Twitter page from December 2, 2021, the entire section on vaccine disinformation is not present with the exception of three references which are forbidden as follows:
1.) The pandemic or COVID-19 vaccines that invoke a deliberate conspiracy by malicious and/or powerful forces.
2.) Vaccines and vaccination programs which suggest that COVID-19 vaccinations are part of a deliberate or intentional attempt to cause harm or control populations.
3.) How vaccines are developed, tested, and approved by official health agencies as well as information about government recommendations.
In fact and as an aside, Twitter is actually guilty of disinformation considering that this is a quote from a Science Brief that appears on the CDC website:
With this example of Big Technology censorship in mind, let's go to the main subject of this posting. A recent open letter which appeared on the British Medical Journal (BMJ) website was submitted by two members of the editorial staff at the BMJ to Mark Zuckerberg. Fiona Godlee and Kamran Abbasi's letter states the following:
"We are writing to raise serious concerns about the “fact checking” being undertaken by third party providers on behalf of Facebook/Meta.
In September, a former employee of Ventavia, a contract research company helping carry out the main Pfizer covid-19 vaccine trial, began providing The BMJ with dozens of internal company documents, photos, audio recordings, and emails. These materials revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety. We also discovered that, despite receiving a direct complaint about these problems over a year ago, the FDA did not inspect Ventavia’s trial sites.
The BMJ commissioned an investigative reporter to write up the story for our journal. The article was published on 2 November, following legal review, external peer review and subject to The BMJ’s usual high level editorial oversight and review."
Here is the November 2, 2021 article mentioned in the open letter:
Here are some quotes from the November 2, 2021 article with my bolds:
"In autumn 2020 Pfizer’s chairman and chief executive, Albert Bourla, released an open letter to the billions of people around the world who were investing their hopes in a safe and effective covid-19 vaccine to end the pandemic. “As I’ve said before, we are operating at the speed of science,” Bourla wrote, explaining to the public when they could expect a Pfizer vaccine to be authorised in the United States.
But, for researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails....
In a recording of a meeting in late September 2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. “In my mind, it’s something new every day,” a Ventavia executive says. “We know that it’s significant.”
In her 25 September email to the FDA Jackson wrote that Ventavia had enrolled more than 1000 participants at three sites. The full trial (registered under NCT04368728) enrolled around 44 000 participants across 153 sites that included numerous commercial companies and academic centres. She then listed a dozen concerns she had witnessed, including:
Participants placed in a hallway after injection and not being monitored by clinical staff
Lack of timely follow-up of patients who experienced adverse events
Protocol deviations not being reported
Vaccines not being stored at proper temperatures
Mislabelled laboratory specimens, and
Targeting of Ventavia staff for reporting these types of problems....
In Pfizer’s briefing document submitted to an FDA advisory committee meeting held on 10 December 2020 to discuss Pfizer’s application for emergency use authorisation of its covid-19 vaccine, the company made no mention of problems at the Ventavia site. The next day the FDA issued the authorisation of the vaccine.
In August this year, after the full approval of Pfizer’s vaccine, the FDA published a summary of its inspections of the company’s pivotal trial. Nine of the trial’s 153 sites were inspected. Ventavia’s sites were not listed among the nine, and no inspections of sites where adults were recruited took place in the eight months after the December 2020 emergency authorisation. The FDA’s inspection officer noted: “The data integrity and verification portion of the BIMO [bioresearch monitoring] inspections were limited because the study was ongoing, and the data required for verification and comparison were not yet available to the IND [investigational new drug].”
Let's go back to the open letter. The authors of the letter note the following with my bolds:
"But from November 10, readers began reporting a variety of problems when trying to share our article. Some reported being unable to share it. Many others reported having their posts flagged with a warning about “Missing context ... Independent fact-checkers say this information could mislead people.” Those trying to post the article were informed by Facebook that people who repeatedly share “false information” might have their posts moved lower in Facebook’s News Feed. Group administrators where the article was shared received messages from Facebook informing them that such posts were “partly false.”
Readers were directed to a “fact check” performed by a Facebook contractor named Lead Stories.
We find the “fact check” performed by Lead Stories to be inaccurate, incompetent and irresponsible.
-- It fails to provide any assertions of fact that The BMJ article got wrong
-- It has a nonsensical title: “Fact Check: The British Medical Journal Did NOT Reveal Disqualifying And Ignored Reports Of Flaws In Pfizer COVID-19 Vaccine Trials”
-- The first paragraph inaccurately labels The BMJ a “news blog”
-- It contains a screenshot of our article with a stamp over it stating “Flaws Reviewed,” despite the Lead Stories article not identifying anything false or untrue in The BMJ article
-- It published the story on its website under a URL that contains the phrase “hoax-alert”
We have contacted Lead Stories, but they refuse to change anything about their article or actions that have led to Facebook flagging our article.
We have also contacted Facebook directly, requesting immediate removal of the “fact checking” label and any link to the Lead Stories article, thereby allowing our readers to freely share the article on your platform."
Here is the key sentence:
"There is also a wider concern that we wish to raise. We are aware that The BMJ is not the only high quality information provider to have been affected by the incompetence of Meta’s fact checking regime. To give one other example, we would highlight the treatment by Instagram (also owned by Meta) of Cochrane, the international provider of high quality systematic reviews of the medical evidence. Rather than investing a proportion of Meta’s substantial profits to help ensure the accuracy of medical information shared through social media, you have apparently delegated responsibility to people incompetent in carrying out this crucial task. Fact checking has been a staple of good journalism for decades. What has happened in this instance should be of concern to anyone who values and relies on sources such as The BMJ."
Here is a screen capture of the entire open letter should it happen to disappear from the internet as things are prone to do in the post-truth era:
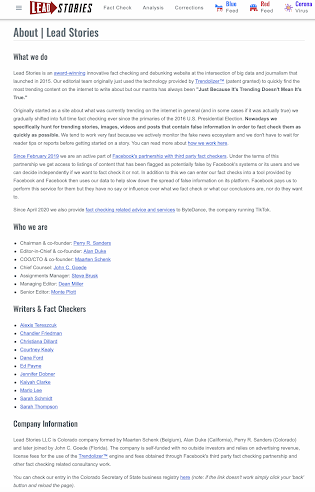
As well, in case you were curious, here is Lead Story's webpage, showing the names of the fact checkers that the company employs, only one of which has any connection to "science" (a BSc in Science) with the majority of the remaining fact checkers having backgrounds in journalism which is clearly does not qualify them to ascertain the scientific validity of any research being provided to them for analysis:
So basically, if we boil it down to its essentials, fact checking is little more than the generation of propaganda that suits the narrative of the company/person that has hired the fact checkers.
It is very clear that, over the past two years, so-called "fact checking" has become laughable. People with absolutely no education or training in medical science are controlling the COVID-19 narrative, using the heavy hand of censorship to negate any viewpoints that do not follow the interests of their employers and those who employ their employers.
Scientific facts have clearly become one of the casualties of the pandemic thanks to the efforts by Big Technology whose mission seems to be to prevent us from learning about the truth as they don't see it.










The post-truth era doesn't only belong to Big Tech and Big Pharma, it belongs to all of us and underlines the importance of focusing (yes independent thought for enlightenment of the greater good) on objective reasoning based on facts over and above emotional hysteria.
ReplyDeletehttps://www.pnas.org/content/118/51/e2107848118
If 'fact-checking' is the idea, the 'alternative' has to be better (more valuable) than the primary source, otherwise it just adds to the noise.
'Information' has never circulated so easily and responsible debunking takes a lot more work than viral-type dissemination of poorly substantiated 'impressions'.