Here is a recent announcement that appeared on the United States government's clinical trials website:
Anthony Fauci's former employer, the National Institute of Allergy and Infectious Diseases or NIAID is sponsoring a Phase 1 trial of a mRNA-LNP (lipid nanoparticle) vaccine for influenza. The vaccine, currently has the catchy name H1ssF 3928 (aka VRC-FLUNPF099-00-VP) and is known as a virus quadrivalent inactivated vaccine. The name of the vaccine is an abbreviation of H1 hemagglutinin stabilized stem ferritin which means that the vaccine uses the stem part of the influenza vaccine as its immunogen rather than the head of the virus which is the target of traditional influenza vaccines.
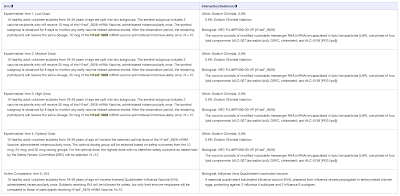
In the trial, the vaccine will be intramuscularly administered to 50 healthy adult volunteers between the ages of 18 and 49 years inclusive. Volunteers will be divided into five groups of ten individuals in five experimental arms. The purpose of the trial is to assess the safety and immunogenicity (i.e. effectiveness) of one dose of H1ssF 3928 at various doses of 10mcg, 25 mpg and 50 mcg as follows:
One of the arms of the trial will be used to compare the antibody responses to H1ssF 3928 with a traditional standard dose of IIv4, a traditional quadrivalent influenza vaccine that is "effective" against four different influenza viruses (two influenza A viruses and two influenza B viruses) that many of us have received over our lifetimes.
Here are more details on how the trial will be conducted:
"Eligible participants will be sequentially enrolled into dosing groups (10 mcg, 25 mcg, and 50 mcg, selected optimal dose) to receive the H1ssF 3928 mRNA Vaccine at the specified dose. A separate group of 10 participants will receive licensed quadrivalent influenza vaccine (IIV4). Subjects receiving IIV4 will be followed for safety but only their immune responses will be compared to those of participants receiving H1ssF 3928 mRNA Vaccine. Dosing of H1ssF 3928 mRNA Vaccine will commence at the lowest dose (10 mcg) and only escalate to the next highest dose if safety concerns are not identified. For each VRC H1ssF 3928 mRNA-LNP (Lipid Nanoparticle) dosing group, the first two subjects enrolled will be considered the sentinel subgroup. After the two subjects in the Low Dose sentinel subgroup are enrolled and given their vaccination, enrollment will then be stopped pending a review of the reactogenicity and safety data collected through Day 3 for both subjects. Approval by the reviewing group will allow for continued enrollment of the remaining Low Dose Group subjects to complete enrollment of 10 participants. After Low Dose Group enrollment is completed, enrollment will be stopped pending an Safety Review Committee (SRC) review of the reactogenicity and adverse event (AE) information through Day 7 and clinical laboratory results through Day 8 for all Low Dose Group subjects. Approval will allow dose escalation and initiation of enrollment of the Medium Dose Group sentinel subgroup. After the two subjects in the Medium Dose sentinel subgroup are enrolled and given their vaccination, enrollment will then be stopped pending a safety review of the sentinel subgroup as specified for the Low Dose Group. Approval will allow for continued enrollment of Medium Dose Group subjects to complete enrollment of 10 participants. After the Medium Dose Group enrollment is completed, enrollment will be stopped pending an SRC review of the reactogenicity and adverse event information through Day 7 and clinical laboratory results through Day 8 for all Medium Dose Group subjects. Approval will allow dose escalation and initiation of enrollment of the High Dose Group sentinel subgroup. After the two subjects in the High Dose sentinel subgroup are enrolled and given their vaccination, enrollment will then be stopped pending a safety review of the sentinel subgroup as specified for the Low and Medium Dose Groups. Approval will allow for continued enrollment of High Dose Group subjects to complete enrollment of 10 participants. After the High Dose Group enrollment is completed, enrollment will be stopped pending an SRC review of the reactogenicity and adverse event information through Day 7 visit and clinical laboratory results through Day 8 for all High Dose Group subjects. Reactogenicity and AE information through Day 7 and clinical laboratory results through Day 8 from the first three dosing groups will guide the selection of an optimal dose group to include an additional 10 subjects who will receive the optimal dose of mRNA-LNP. The primary objective of this study is to assess the safety of a single dose of VRC H1ssF 3928 mRNA-LNP vaccine administered IM in healthy adults, 18-49 yrs, at doses of 10 mcg, 25 mcg, and 50 mcg. The secondary objective of this study is to assess serum antibody responses to a single dose of VRC H1ssF 3928 mRNA-LNP vaccine administered IM in healthy adults at doses of 10 mcg, 25 mcg, and 50 mcg in comparison to a standard dose of IIV4."
Here are the primary outcome measures:
1.) Occurrence of any adverse events of special interest (AESIs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 366 ]
2.) Occurrence of any influenza-like illnesses (ILIs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 366 ]
3.) Occurrence of any medically attended adverse events (MAAEs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 366 ]
4.) Occurrence of any new-onset chronic medical conditions (NOCMCs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 366 ]
5.) Occurrence of any serious adverse events (SAEs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 366 ]
6.) Occurrence of any unsolicited adverse events (AEs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 28 ]
7.) Occurrence of clinical laboratory adverse events (AEs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 57 ]
8.) Occurrence of solicited reactogenicity adverse events (AEs) with VRC H1ssF_3928 mRNA-LNP vaccine. [ Time Frame: Day 1 through Day 14 ]
Both local and systemic adverse events will be assessed
The study had an estimated starting date of May 12, 2023 although the website claims that recruitment has not begun and a final data completion date of March 15, 2024 for the primary outcome measure.
Better line up folks.







"mRNA-LNP (liquid nanoparticle)" is LIPID nanoparticle (are spherical vesicles made of ionizable lipids)
ReplyDeleteThanks. Fixed. I was a victim of auto spellchecking.
Delete