With the exception of politicians, health care officials and the mainstream media who seem to have very little knowledge about the mRNA technology being used in the COVID-19 vaccines, the history of the use of mRNA in vaccines has been fraught with issues. Nonetheless, Moderna, one of the three main players in the mRNA space is going full bore on the use of this technology for other uses.
Here is a brief article that looks at history of the technology as quoted from the Bloomberg School of Public Health at Johns Hopkins University:
Here are some quotes:
"Messenger RNA, or mRNA, was discovered in the early 1960s; research into how mRNA could be delivered into cells was developed in the 1970s. So, why did it take until the global COVID-19 pandemic of 2020 for the first mRNA vaccine to be brought to market?
The early years of mRNA research were marked by a lot of enthusiasm for the technology but some difficult technical challenges that took a great deal of innovation to overcome.
The biggest challenge was that mRNA would be taken up by the body and quickly degraded before it could “deliver” its message—the RNA transcript—and be read into proteins in the cells.
The solution to this problem came from advances in nanotechnology: the development of fatty droplets (lipid nanoparticles) that wrapped the mRNA like a bubble, which allowed entry into the cells. Once inside the cell, the mRNA message could be translated into proteins, like the spike protein of SARS-CoV-2, and the immune system would then be primed to recognize the foreign protein.
SO, WHAT HAPPENED ONCE THEY FIGURED OUT THIS TECHNOLOGY?
The first mRNA vaccines using these fatty envelopes were developed against the deadly Ebola virus, but since that virus is only found in a limited number of African countries, it had no commercial development in the U.S.
THEN COVID-19 HIT … WHAT HAPPENED THEN?
Remember, the COVID-19 pandemic spurred manufacturers to develop dozens of potential vaccines against SARS-CoV-2 and brought tremendous increases in funding. Some of those vaccines used traditional methods involving adenovirus as the spike protein delivery system—such as the Johnson & Johnson vector vaccine.
Thanks to decades of research and innovation, mRNA vaccine technology was ready. With COVID, this technology got its moment and has proven to be extremely safe and effective. Pfizer’s COVID-19 vaccine is the first mRNA product to achieve full FDA approval in the U.S."
Proven to be extremely safe and effective? Hmmm, let's see about that. Given the widespread government and public health approval of the mRNA technology, it is interesting to note that Moderna's mRNA-1273 vaccine trial isn't scheduled for completion until December 29, 2022 as shown here:
Nonetheless, Moderna, a company with no product sales revenue until 2020 when its COVID-19 vaccine started rolling out and massive losses attributable to common shareholders as shown here:
...is going all in for mRNA technology.
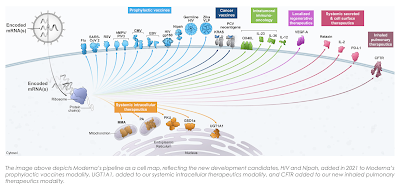
Here is a table and a graphic showing Moderna's product pipeline which solely focuses on mRNA:
The only product which is currently commercial is its mRNA-1273 COVID-19 vaccine. While most of its other products are in preclinical or Phase 1 trials including its influenza vaccines, the company's CMV (human cytomegalovirus) vaccine, mRNA-1647, is the second vaccine to undergo Phase 3 trials in the company's history. With RSV being the new bogeyman in the world of health, it is interesting to note that the company's mRNA-1345 RSV vaccine is currently in Phase 3 trials with completion projected for November 30, 2024 as shown here:
One has to wonder how long it will be before the FDA approves the mRNA-1345 vaccine given the growing concern about the rapid spread of respiratory Syncytial Virus through the population in recent weeks.
I find it fascinating that Moderna is putting all of its "eggs" into the mRNA "basket", particularly given that the trials for its headline product, the mRNA-1273 COVID-19 vaccine, are not yet complete, meaning that it is unclear what potential health issues could be related to the widespread use of this technology over the medium- and long-term. As well, the fact that Big Pharma is able to conduct its Phase 3 trials at the same time as the vaccines are being administered to the public plays well to shareholders since it helps profitability by reducing the high cost of trials during the development phase of new pharmaceuticals.










No comments:
Post a Comment